Highfield Biopharmaceuticals Co. launches Phase 1 of its HF1K16 clinical study; a new immunotherapeutic weapon in the fight against solid tumours
Recently, one of the resident start-ups at the China Australia Medical Innovation Park,
Highfield Biopharmaceuticals Co. successfully launched the assessment of injectable liposome wrapped in full trans-formic acid (ATRA) HF1K16 as a single drug to treat local late-stage or metastatic solid tumour tolerance, dose-limiting toxicity, pharmacokinetics and initial effectiveness of open, multi-dose, dose-increasing Phase 1 study in Fudan University’s affiliated Huashan Hospital.
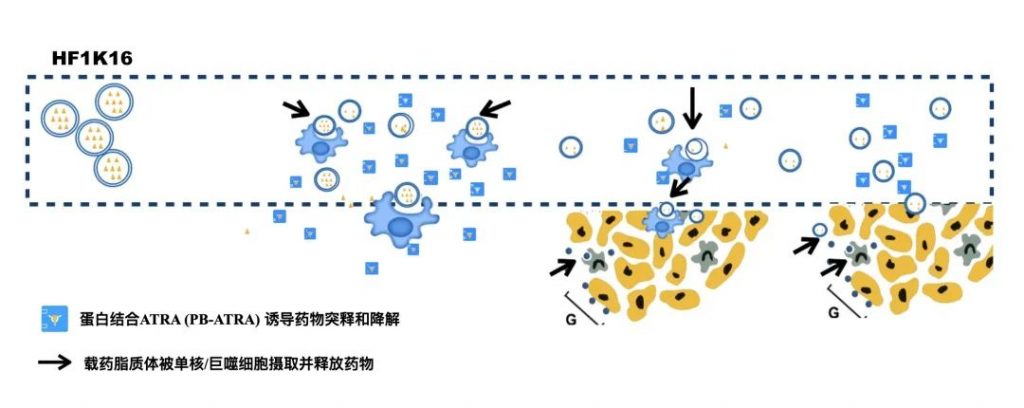
HF1K16 is an innovatively formulated product developed independently by Highfield, based on lipid self-assembly technology, and is also the first global all-trans paracetaphate system agent developed by active carrier technology. This promotes the maturity and differentiation of myelin inhibitory cells (MDSC) in tumour patients by improving the apparent solubility of insoluble compounds, improving system exposure and pharmacokinetic characteristics, and promoting the maturation and differentiation of myelin inhibitory cells (MDSC) in tumour patients to provide an impetus for the immune system’s removal of tumour cells. After launching Phase 1 trials of HF1K16 in the United States, it has been confirmed in a study involving healthy volunteers that after a single dose of the drug, its half-life was greatly extended, whilst simultaneously displaying a better safety profile.
The key role of MDSC in tumour immunosuppression has been in great focus, although currently, there is no clear market for immunotherapy drugs targeting MDSC cells. The main purpose of this study is to evaluate the tolerance of HF1K16 monotherapy, to determine its dose-limiting toxicity, its maximum tolerated dose (MTD), if any, to explore its initial anti-tumour effect, and to evaluate the relationship between exposure of HF1K16 and changes in peripheral blood lymphocytes.
drug formulation
This study has been approved by the State’s Department of Drug Administration (lot number: 2020LP00771) and approved by the Huashan Hospital Ethics Committee, program number: HF1K16-101.
"Biopharmaceutical sciences innovation provides tremendous potential for breakthrough and to power China's continuous economic growth for the next decade. Research-oriented clinical hospitals and national medical centres should resolutely support the development and translation of novel drug development in China," said Jinsong Wu, principal investigator of this project, who chaired the launch.
“It is the everlasting goal and purpose of all researchers to be able to proclaim that they have been on the journey of successfully commercializing a novel drug, accompanying its path from bench to bedside, from preclinical to clinical phase research, before finally launching in the market, for the benefit of all patients.”
Jinsong Wu, Principal Investigator
Director Wu is a distinguished department leader of Shanghai’s public health system, professor at Fudan University and deputy director of the Institute of Neurosurgery, deputy director of Huashan Brain Glioma Clinic, and is currently the Chairman of the Committee of Neuro-Oncology of Shanghai’s Anti-Cancer Association, the Standing Committee of the Neuro-Oncology Professional Committee of the China Anti-Cancer Association and the Head of the Brain Glioma Group.
Hangzhou Highfield Biopharmaceutical Co., Ltd
Hangzhou Highfield Biopharmaceutical Co., Ltd. (Highfield Bio) is a leading innovator in lipid drug research and development, focusing on the treatment of cancers and autoimmune diseases to meet the huge clinical needs of both the domestic Chinese and global markets. Highfield Bio's own research pipeline covers small molecules, antibodies and gene therapy drugs, whilst they have also established an integrated platform to support the development of new lipid drugs, including preclinical evaluation, process development, translation research, clinical development and production.
Currently, Highfield Bio has a research and development centre and complex injection production workshop covering 3000 square meters in Binjiang’s Minsheng Pharmaceutical Industry Park, and a research and development laboratory and pilot workshop of 300 square meters in Zijingang Campus of Zhejiang University. The company's self-developed HF1K16 liposome injections are being tested simultaneously in China and the United States; HF158K1 antibody liposomes are about to embark on clinical trials, with plans to conduct simultaneous studies in Australia, China, the United States and Europe. In addition, HF5050 and HFG101, along with other new-generation antibodies and nucleic acid-lipid nanoparticle drugs are also in the development pipeline.
Highfield Bio is committed to building a world-class biopharmaceutical company with the core values of "敦和、精思、予能". The company's mission is to rely on innovative lipid technology, with superior product development capabilities and speed to market, to provide better quality cost-controlled novel drugs and therapies to address the unmet clinicasl needs of patients.



文章评论(0)